Mycoplasma Elimination in Cell Culture
By Abdos
Introduction
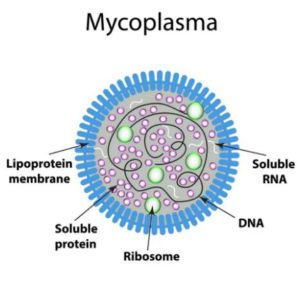
Mycoplasma contamination in Cell Culture is a major concern and it can affect cell growth, cell morphology and may also result in cell death. Mycoplasma is a type of bacteria that does not have a rigid cell wall and falls in the category of Mollicutes, having a size range of 0.3 – 0.8 microns. By virtue of their nature, Mycoplasma is able to squeeze themselves through the pores of even sterilization-grade filter membranes.
Mycoplasma contamination is accidentally introduced by humans when they speak or breathe inside the Cell Culture facility without proper PPEs. Another source of Mycoplasma contamination is through the Sera that is added during the media preparation. So, it is mandatory to sterilize the cell culture media one final time before it is being used for that day’s work. Once detected, frequent checks have to be in place to rule out Mycoplasma contamination inside the facility.
Since Sera and many proteins supplements that are added to the media are heat-labile, an ideal method for media sterilization would be to follow a multiple stage of filtration method based on the turbidity of the media which is being sterilized. The first stage is to clarify the turbidity from the media using a 0.45 micron filter, followed by a 0.22 micron filter to eliminate bacteria and endotoxins. And finally to ensure elimination of Mycoplasma in the media, a 0.1 micron filter must be used. The filtration device being used must be pre-sterilized with the filter in place and should be freshly opened inside the hood where the media is being sterilized.
ABDOS Life Science has introduced Biofill–a bottle top filtration device that is built with filtration funnel and a receiver bottle in a sealed pack (gamma irradiated). The device is available in various sizes having filter pore sizes ranging from 0.45 micron, 0.22 micron and 0.1 micron. The device is also conveniently available with the filtration funnels alone, to be used along with pre-sterilized receiver containers of various sizes depending on the volume of the media.



Conclusion: Hence a lab personnel who handles the cell culture work must wear proper PPE, conduct experiments inside a validated Cell Culture facility and make periodic checks to rule out Mycoplasma contamination. Cell Culture media with heat-labile components must be finally filter sterilized preferably with a 0.1 micron, pre-assembled filtration device that is gamma-irradiated. If Mycoplasma Contamination is not controlled it can lead to loss of productive time, repetition of all the cell culture procedures and thereby cost the scientist dearly.